Specific heat capacity of any substance is defined as the amount of heat required to change the temperature of a unit mass of the substance by 1 degree The below-mentioned. It is proportionality constant between the heat and temperature.
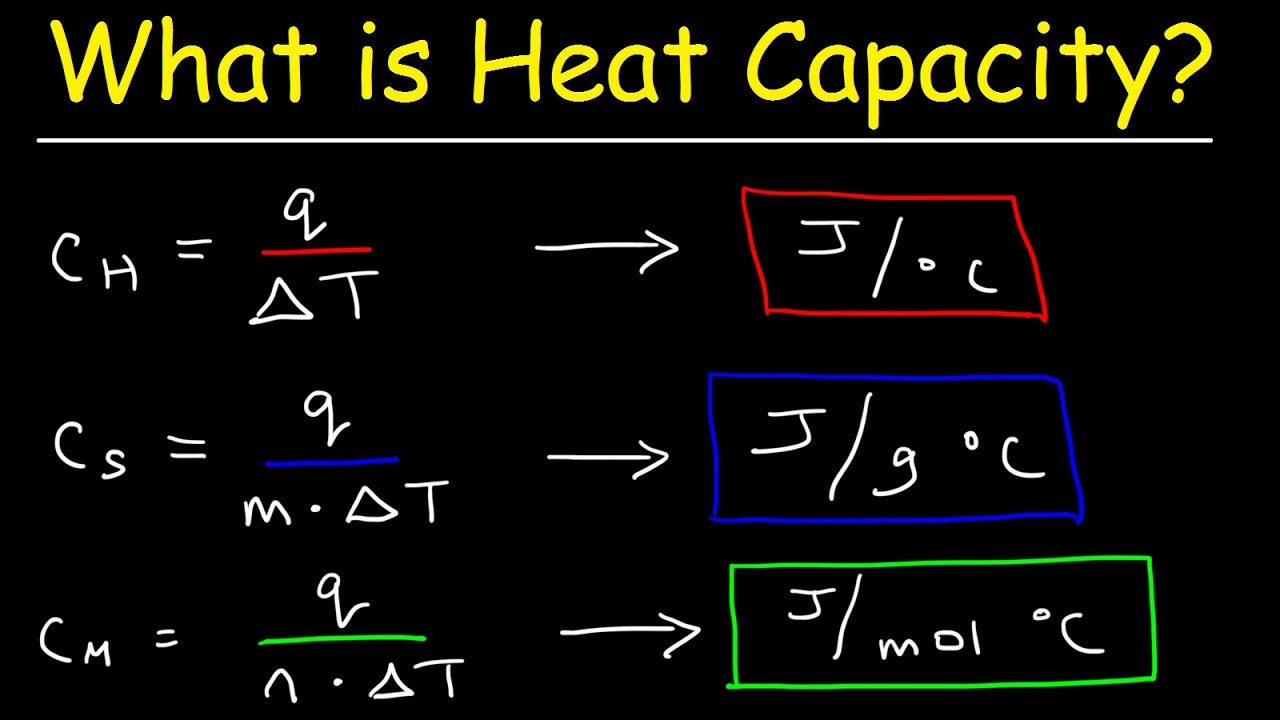
What Is The Difference Between Specific Heat Capacity Heat Capacity And Molar Heat Capacity Youtube
Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature.
. In thermodynamics the specific heat capacity symbol cp of a substance is the heat capacity of a sample of the substance divided by the mass of the sample also sometimes referred to as massic heat capacity. The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the temperature increases or decreases 1. Specific Heat Capacity Formula.
The specific heat capacity of a substance is the amount of energy needed to change the temperature by 1 unit of material of 1 kg mass. The intensive properties cv and cp are defined for pure simple. The SI unit of specific heat and specific heat.
General Physics the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. The meaning of SPECIFIC HEAT is the heat in calories required to raise the temperature of one gram of a substance one degree Celsius. This means that it takes 4200 J to raise the temperature of one kg of water by 1 C.
Informally it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The amount of heat energy required to change the temperature of any substance is given by Q mcΔt where m mass of the substance c. For example the heat required to raise the tempe.
The SI unit of heat. Heat capacity for a given matter depends on its size. The SI unit of specific heat capacity is joule per kelvin per kilogram Jkg K.
Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree Celsius. The definition of specific heat capacity of any substance is the quantity of heat required to change the temperature of a unit mass of the substance by 1 degree This is articulated as. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C.
Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius C. Calculating thermal energy changes The amount of. The specific heat capacity.
Water has a high specific heat meaning it takes. Specific heat or specific heat capacity is a property related to internal energy that is very important in thermodynamics. The heat capacity of a substance per unit mass is called the specific heat capacity cp.
Of water is 4200 joules per kilogram per degree Celsius JkgC.
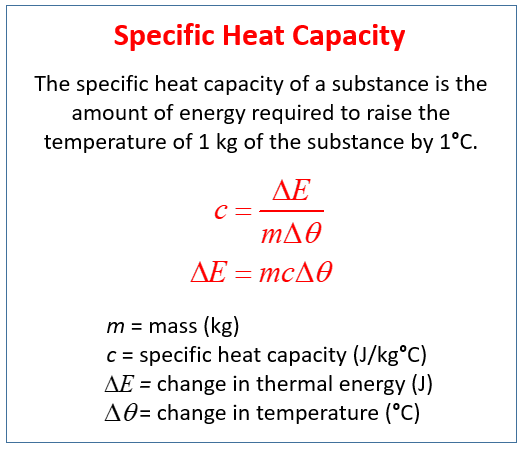
Specific Heat Capacity Experiment Video Lessons Examples Step By Step Solutions

Specific Heat Capacity Definition Molar Specific Heat Videos Examples
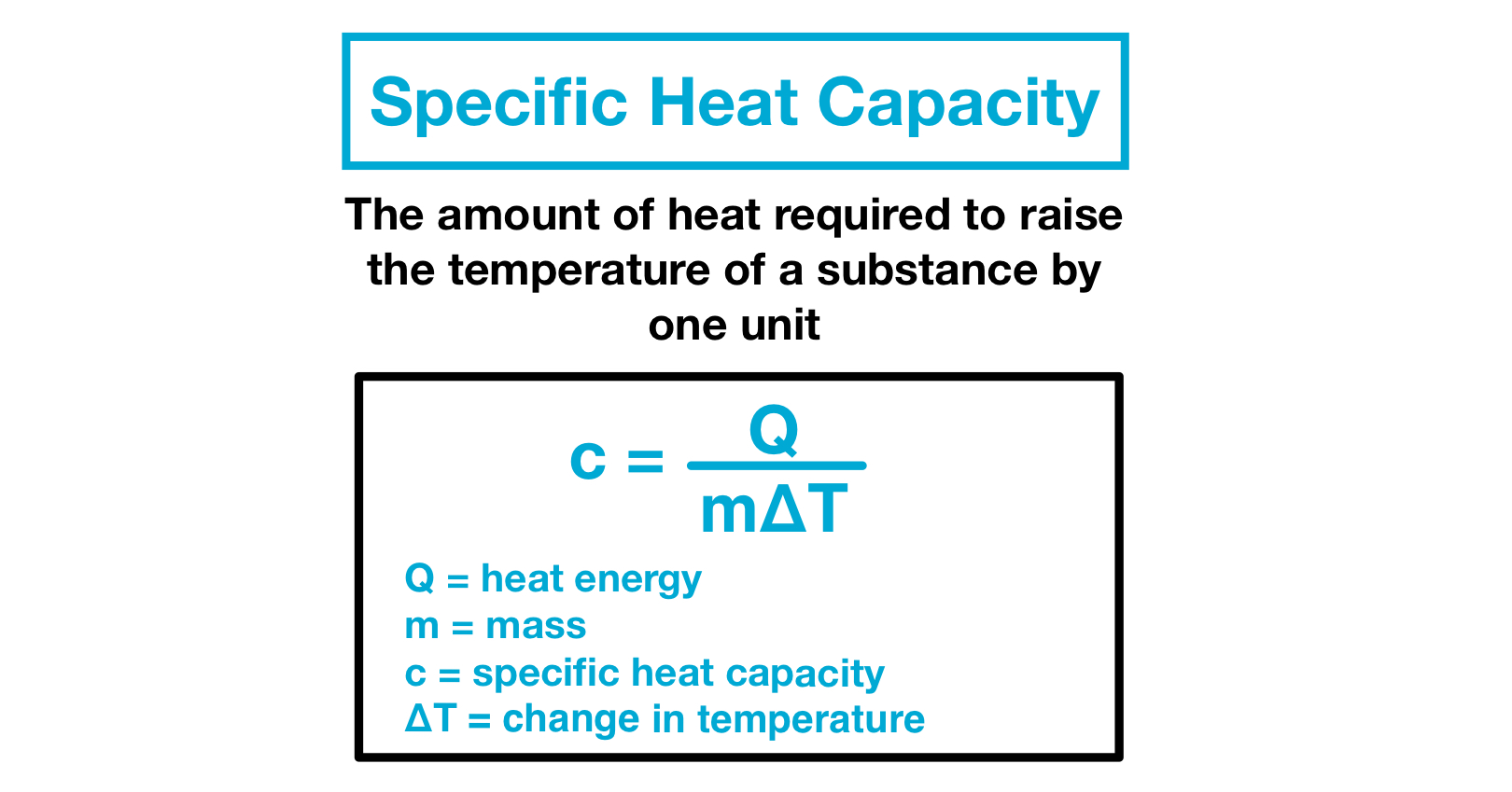
0 Comments